|
|
|
DC Discharge
Often, a d.c.discharge is necessary to produce the
types of molecules studied in our laboratory. The discharge can result
in a brightly colored plasma due to electronic excitations or
chemilluminescence of the metal being heated. This
effect is observed through a quartz window located above or beside the
oven.
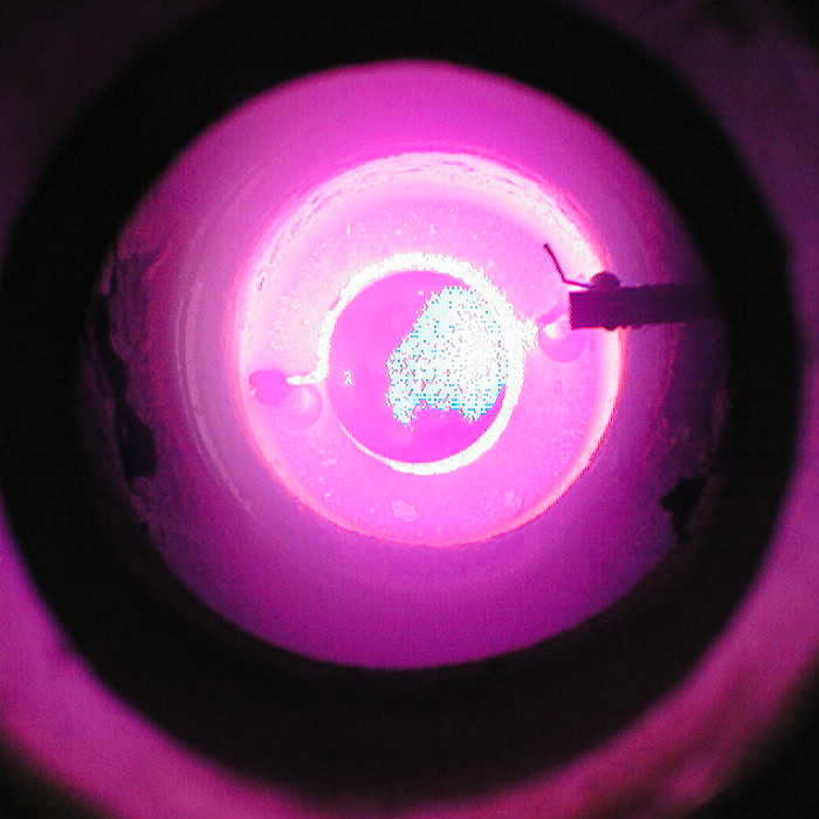
Below are views of (from left to right) the atomic
emission of alkaline-earth metals, magnesium, calcium, and strontium
from above the oven.
  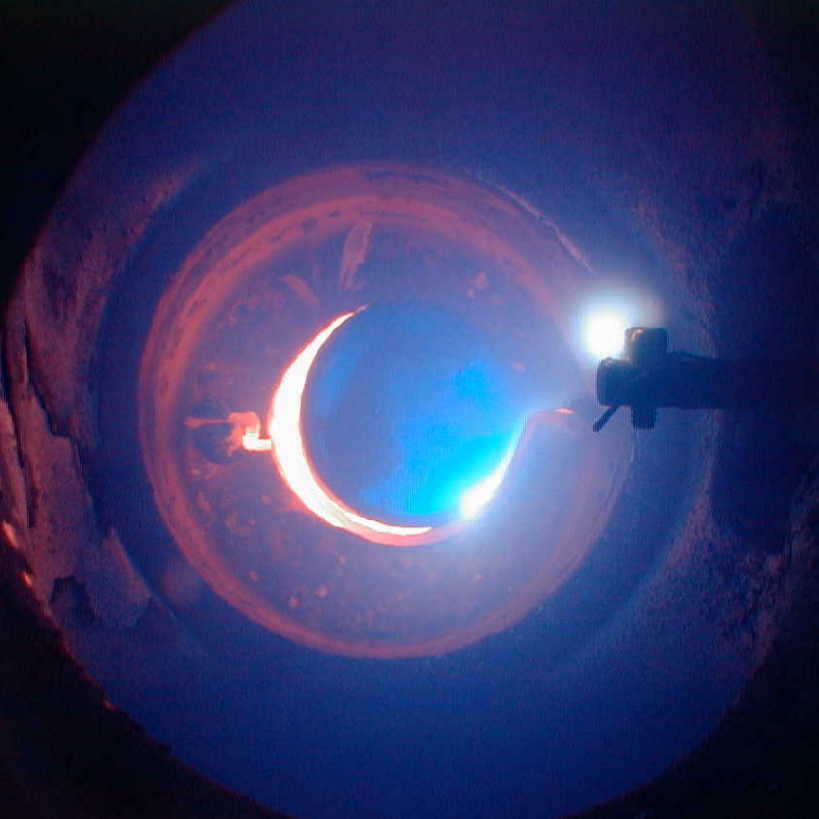
On the left is a view into the reaction cell from the
side; on the right is a view of sodium atomic emission.
AC Discharge
An a.c. discharge is used to produce the ions and
radicals studied in our velocity modulation spectrometer. This
discharge is created between two ring discharge electrodes and causes
brightly colored plasma due to electronic excitations or
chemilluminescence of the gases in the glass cell. This
effect can be see by looking through the glass cell.
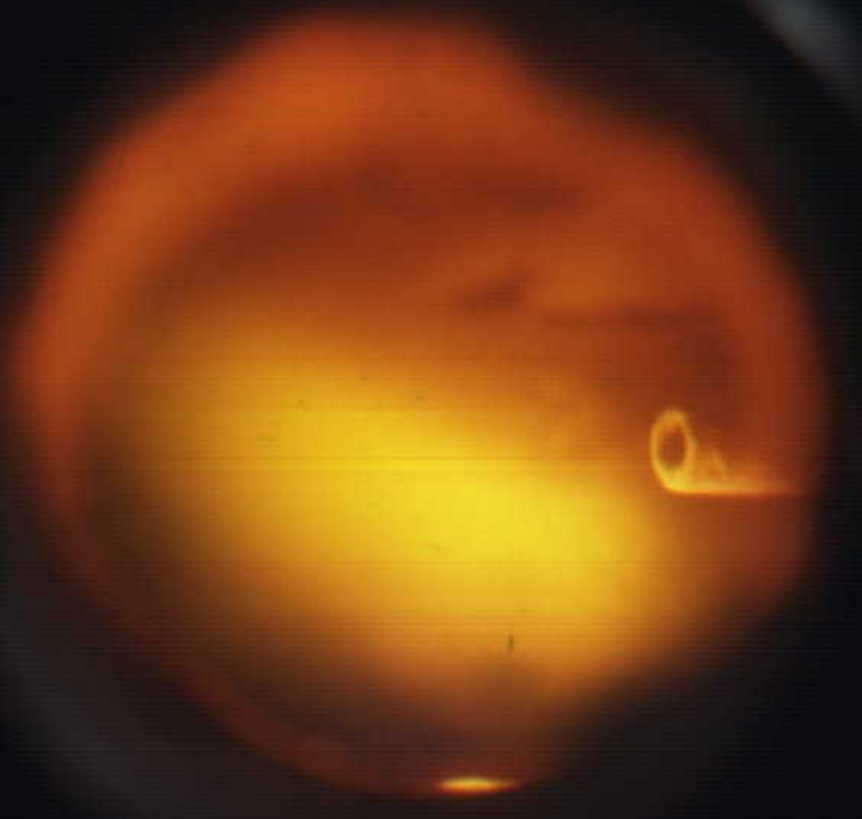
Below are views of (from left to
right) the atomic emission of Ar and the molecular emission of CO+,
created by discharging CO.
Below is a view of the atomic emission of Ti, created by discharging
TiCl 4.
 |










